Past Issues
Palliative Systemic Treatment of Advanced Merkel Cell Carcinoma in the Pre-Immunotherapy Era: A Retrospective, Single-Center Analysis of Patients with An Orphan Neuroendocrine Malignancy
Patrick Schöffski*, Gitte Moors, Paul Clement, Herlinde Dumez, Oliver Bechter
Department of General Medical Oncology, Laboratory of Experimental Oncology, Leuven Cancer Institute, University Hospitals Leuven, KU Leuven, Belgium
Corresponding author: Patrick Schöffski, Department of General Medical Oncology, Laboratory of Experimental Oncology, Leuven Cancer Institute, University Hospitals Leuven, KU Leuven, Belgium.
Received: May 28, 2019 Published: June 27, 2019
ABSTRACT
Objectives: To evaluate the efficacy of systemic treatments in patients with advanced Merkel cell carcinoma (MCC) prior to the introduction of immune checkpoint modulation. Methods: Fifty-nine patients were treated at the University Hospitals Leuven between 1999 and 2012. Seventeen had advanced disease incurable by radiotherapy and/or surgery, received one or multiple systemic treatments and were included in this retrospective analysis. Results: MCC is a chemotherapy-sensitive tumour with an objective response rate of 63% to first-line chemotherapy. The combination of cisplatin or carboplatin with etoposide was the most frequently used regimen (n=13) with responses seen in 69% of patients. The median progression-free survival after first-line chemotherapy was 8 months. Eight patients received second line chemotherapy with gemcitabine, taxanes or vinca alkaloids with a response rate of 25%. The median overall survival since start of first line chemotherapy was 13 months. Conclusions: A considerable proportion of patients with MCC fails local treatments and requires systemic therapy. Advanced MCC is a chemotherapy-sensitive disease with high response rates. The poor overall survival achieved with chemotherapy supports the need for novel systemic strategies, such as the routine implementation of immunologic treatment approaches. Immune checkpoint modulation is complementary to chemotherapy, and should be further developed as single agent, in sequence or in combination with other biological or cytotoxic therapy.
Keywords: Merkel cell carcinoma, Chemotherapy, Prognosis
INTRODUCTION
Merkel cell carcinoma (MCC) is a rare, aggressive skin malignancy [1]. The true incidence in Europe is currently unknown, as these tumours are not recorded separately from non-melanoma skin cancers in most registries. There is a 3-fold increase in the number of MCC diagnoses over the past years, likely due to better screening and identification of high-risk populations [1,2].
MCC belongs to the broader family of neuroendocrine malignancies, characterized histologically by dense-core neurosecretory granules, atypical mitosis and high apoptotic activity on microscopy. Immunohistochemically, MCC stains positive for cytokeratin 20 and 7, chromogranin A, neuron-specific enolase, synaptophysin and CD 65 [1,3,4]. In historical series, MCC had an estimated overall 5-year survival rate of 62% [5]. The average age at diagnosis is 69 years. Ultraviolet radiation and immunosuppression increase the risk of MCC, as with other skin tumors [6-10], underlining the potential role of the immune system in this disease. Probably UV radiation induces the oncogenic potential of the common Merkel cell polyomavirus (MCV), which is ubiquitous in humans. While the virus normally does not enter the human genome, it does integrate with the genome in Merkel cell carcinoma, where it gives rise to the production of oncoproteins and induces mutations [11]. Since MCC occurs predominantly in sun-exposed skin in the head and neck in older individuals, UV-induced mutations may trigger viral DNA integration in the tumour genome. MCC is most commonly staged according the following classification: stage I, localized disease (IA ≤ 2 cm, IB > 2 cm); stage II, regional lymph node involvement; stage III, distant metastasis [7]. In 2010 a new staging system was proposed which divides patients into 4 groups [6,7].
Most MCC patients present with a localized tumour (Stage I). The 5-year survival of these patients is in the range of 64% [6]. Surgery with wide local excision is the generally accepted standard treatment. Mohs micrographic surgery is an option with comparable local recurrence rates [12]. Some authors advocate postoperative radiotherapy, since MCC is known to be radiotherapy-sensitive [6]. Involvement of lymph nodes (Stage II) is associated with worse prognosis (5-year survival around 47%), since nodal spread is associated with a risk of developing distant metastasis [6]. Although only retrospective data are available and results are conflicting, a database analysis showed an improvement in overall survival with adjuvant radiotherapy compared to surgery alone [13]. Treatment standards for stage II MCC usually include surgery and radiotherapy to the affected sites [7]. Adjuvant chemotherapy is a hypothetical option for patients at high risk of recurrence, although this has never been studied prospectively and most studies were using chemo-radiation, making the effect of adjuvant chemotherapy even more difficult to assess [14]. Until recently there was no real established treatment standard for metastatic MCC. Theoretical options included chemotherapy, radiotherapy and surgery in individual cases. MCC is generally perceived by oncologists as a chemotherapy-sensitive neuroendocrine tumour. The reported evidence for the use of cytotoxic agents in advanced MCC is very limited, and a variety of chemotherapeutic agents are used in clinical routine. The choice of treatment is commonly extrapolated from the experience in other neuroendocrine malignancies, such as in small cell lung cancer [3,8]. The true role and efficacy of chemotherapy in MCC remains elusive due to the absence of prospective controlled trials. This lack of evidence with regard to efficacy and outcome is adding to the fact that most chemotherapy combinations currently applied for MCC can pose considerable and even irreversible toxicity to the patient. Knowledge over the role of chemotherapy in MCC is therefore the basis for further improving treatment concepts in this rare aggressive disease.
Our report summarizes the retrospective experience of the University Hospitals in Leuven with the systemic treatment of advanced, incurable MCC over a period of 13 years prior to the immunotherapy era. The purpose of this report is to document the role of cytotoxic therapy in the absence of historical prospective evidence, and to underline the unmet medical need for novel, safe and effective treatments for this orphan disease.
MATERIALS AND METHODS
After approval of this project by the independent Ethics Committee of the University Hospitals in Leuven, we performed a retrospective analysis of all electronic patient files referring to the diagnosis of MCC in our medical center. We focused on those cases where systemic therapy was administered at any given time during the course of the disease. Due to the retrospective nature of this analysis we did not collect individual informed consent. Data were handled confidentially according to Belgian privacy laws.
A total of 59 MCC patients were treated between 1999 and 2012. Seventeen of them received one or multiple systemic treatments. They were selected irrespective of other treatments received, such as surgery and/or RT. The following data were collected and entered into a specific data base: age, gender, cancer history, primary site of MCC, treatment and outcome (response, survival). Progression-free survival was defined as the time from start of systemic treatment until objective tumor progression or death. Response assessment with CT scans was done according to established clinical routine, response data were retrieved from the patient files but not verified by independent review. RECIST was the most commonly used tool for response assessment. Survival durations were evaluated from both the date of first diagnosis of MCC and the date of start of first line systemic treatment until the date of last survival update or documented death. Overall survival was estimated using Kaplan-Meier analysis.
We supplemented our analysis with a literature search on this orphan malignancy. We reviewed articles published between 1990 and 2018, and discuss the emerging role of immune checkpoint modulation in this disease.
RESULTS
Patient characteristics
Seventeen of 59 patients (29%) received systemic therapy for advanced, incurable MCC, including 10 male (58.8%) and 7 female (41.2%) patients with a mean age at time of diagnosis of 64 years (range 49-82 years). Only 4 patients were diagnosed before the age of 55 years. Two underwent organ transplantation in the past, namely a liver and kidney transplantation. They developed MCC 3 and 20 years after being transplanted. Four patients had a history of another malignancy prior to the diagnosis of MCC (1 neuroendocrine carcinoma of the stomach, 1 endometrial cancer, 2 breast cancers and 1 basal-cell skin carcinoma). A total of 11 out of 17 patients presented with localized MCC at initial diagnosis, 4 had positive lymph nodes and two patients had synchronous metastatic disease. The lower limbs were the most common primary localization (47%). An overview of the site of disease and distant metastasis is shown in (Tables 1 and 2).
Table 1: Characteristics of MCC patients treated with systemic therapy at the University Hospitals in Leuven.
|
Patient Characteristics |
n |
% |
|
Gender |
||
|
Male |
10 |
59% |
|
Female |
7 |
41% |
|
Age, years (mean, 64) (range 49-82) |
||
|
< 60 |
8 |
47% |
|
≥ 60 |
9 |
53% |
|
Primary site |
|
|
|
Head and neck |
3 |
18 |
|
Upper extremity |
1 |
6 |
|
Lower extremity |
8 |
47 |
|
Trunk |
1 |
6 |
|
Unknown |
4 |
24 |
|
Site of metastasis |
||
|
Lymph nodes |
10 |
59% |
|
Skin |
9 |
53% |
|
Bone |
4 |
24% |
|
Liver |
2 |
12% |
|
Lung |
3 |
18% |
|
Pleura |
1 |
6% |
|
Brain |
1 |
6% |
|
Small bowel |
1 |
6% |
|
Stomach |
1 |
6% |
|
Larynx |
1 |
6% |
|
Pericard |
1 |
6% |
|
Parotis |
1 |
6% |
|
Pancreas |
1 |
6% |
|
Peritoneum |
1 |
6% |
|
Stage at initial diagnosis |
||
|
I |
11 |
65% |
|
II |
4 |
24% |
|
III |
2 |
12% |
Table 2: First-line chemotherapy.
|
Regimens |
n |
Objective response* |
Mean survival (months)** |
|
All |
16 |
63% |
43
|
|
Single agent |
3 |
33% |
28 |
|
Combination chemotherapy |
13 |
69% |
46 |
|
With platinum |
13 |
69% |
46 |
|
With etoposide |
14 |
64% |
43 |
|
With alkylating drugs |
5 |
80% |
86 |
|
Other regimens |
1 |
0% |
11 |
|
* Objective response defined as percentage of patients achieving a complete or partial response ** Mean overall survival since start of systemic treatment (months) |
|||
TREATMENT
Initial surgery: A wide excision of the primary tumor was performed in 11 of the 17 patients. Negative margins were obtained in 8 (73%) cases. In 4 patients the primary site was unknown; two of them underwent a lymph node resection followed by radiotherapy.
Radiotherapy: Fifteen (88%) patients received radiotherapy to different sites of disease. In 10 patients, this was part of the initial treatment with 6 patients receiving adjuvant RT after surgical resection. Two were initially treated by radiotherapy alone. Two patients received radiotherapy combined with chemotherapy. Radiotherapy was used as salvage treatment at time of relapse after initial treatment in 12 patients.
Chemotherapy: A total of 14 patients (82%) had distant metastasis at the beginning of their systemic treatment; only 3 patients (18%) underwent chemotherapy for regional lymph node metastasis. The median time from first diagnosis until start of systemic therapy was 8 months (range 0-141). In total, 20 different treatment regimens were used or first and subsequent lines of therapy, illustrating the poorly standardized treatment pattern of MCC due to the absence of validated standards. Among 17 treated patients, platinum-containing schedules and topoisomerase inhibitors were given in 16 cases each (94%), alkylating drugs in 8 cases (47%), anthracyclines were given to 4 patients (24%), antimetabolites in two cases (12%) and other cytotoxic compounds in 4 patients (24%). The radionuclide Yttrium and the somatostatin analog lanreotide were used in only three patients.
All but one patient received first-line systemic treatment for at least 2 treatment cycles or a minimum of one month oral treatment. The oral agents used as first-line treatment were the mammalian target of rapamycin inhibitor everolimus and the topoisomerase II inhibitor etoposide.
Response to first-line chemotherapy was assessed in 14 out of 16 patients (86%). The objective response rate (defined as complete and partial responses) was 63% (10 of 16). Three patients received single agent chemotherapy with an objective response rate of 33%, while 69% of patients who received combinations had a response to the first-line systemic treatment.
The median progression-free survival after first-line chemotherapy was 8 months (range 1-146). The most commonly used first-line systemic treatment was the combination of a platinum compound with etoposide. In these patients, objective responses were seen in 9 out of 13 patients, with an objective response rate of 69%.
The objective response rate to second-line chemotherapy was 25% (2 of 8). Among 3 patients who received third-line chemotherapy, two (66%) responded. One patient received forth-line chemotherapy and had stable disease.
Three patients in our series achieved a complete response with systemic therapy:
Patient 76941111 was 53 years old at first diagnosis of MCC, which was treated by surgery and adjuvant radiotherapy. He relapsed within half a year after initial surgery with a hematogenic laryngeal metastasis. He was treated systemically with cisplatin/etoposide/ifosfamide and achieved a complete response, which was consolidated by radiotherapy. The patient later relapsed and received palliative treatment with etoposide. He died 15 years after the first diagnosis of MCC due to further disease progression.
Patient 82717703 was a 53 year-old female with diagnosis of MCC and Alport syndrome as described above. She had a complete response to carboplatin/etoposide. She died within 10 months after the initial diagnosis of metastatic MCC.
Patient 71080311 was a 55 year-old female at the diagnosis of locally advanced MCC. Within two years she developed lymph node and skin metastasis. In parallel with the treatment and follow-up of this disease she developed breast cancer at the age of 56 years and a basal cell carcinoma of the skin at the age of 76 and 77 years. Upon treatment with cisplatinum/etoposide she achieved a complete response of her metastatic MCC. She remained in complete response for 13 years of follow-up and is alive 29 years after the initial diagnosis of MCC.
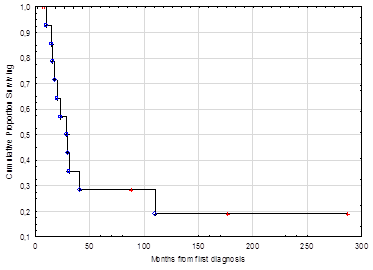
Survival: Survival data of 14 patients are available; the others were lost to follow-up. The 2-year overall survival was 59%, at 5 years 29% of patients were still alive. The median duration of overall survival since first diagnosis was 29 months (range, 8-287) (Figure 1). The median overall survival since the start of chemotherapy was 13 months (range, 1-170). Patient, tumour and treatment characteristics and survival are summarized in Table 1.
Figure 1: Kaplan-Meier survival curve which shows the overall survival for patients with MCC since the time of first diagnosis.
Treatment morbidity: Hematological toxicity and gastrointestinal intolerance were the most commonly reported adverse effects. Five patients required blood or platelet transfusions. Febrile neutropenia was reported in 3 cases. Three treatment associated deaths were reported, all due to septic shock. One patient developed thoracic zoster. One case of ototoxicity was reported, likely related to the treatment with cisplatin (Table 3).
Table 3: Prognostic factors in patients with MCC.
|
Characteristics |
n |
range |
Median survival (months) |
|
Stage at time of diagnosis Stage I Stage II Stage III |
11 4 2 |
(1-170) (4-11) |
23 7.5 63.5 |
|
Stage III |
(15-112) |
||
|
Stage at start of chemotherapy Stage II Stage III |
3 14 |
(1-11) (4-170) |
11 14 |
|
Gender Male Female |
10 7 |
(4-170) |
13 13 |
|
Female |
(10-142) |
||
|
Age at time of diagnosis <60 years ≥60 years |
8 9 7 |
(49-82) |
28 8 |
|
Primary site Head and neck Extremity Trunk Unknown |
3 9 1 4 |
(12-23) (4-142) (5)
|
15 33 5 9.5 |
|
Time between initial diagnosis and metastasis < 6 months ≥ 6 months |
6 11 |
|
24 11 |
|
Response to first-line chemotherapy CR PR MR-SD PD |
3 7 1 3 |
(10-170) (7-112) (4) (5-86) |
142 13 4 33 |
|
CR: Complete response; PR: Partial response; MR: Minor reponse; SD: Stable disease; PD: Progressive disease |
|||
DISCUSSION
We explored the use of chemotherapy in patients with MCC in our tertiary care center by assessing patient and treatment characteristics and treatment outcome. The analysis was purely descriptive due to the relatively low number of patients. Additional limitations of this analysis are the heterogeneity of the chemotherapy regimens used and the retrospective character of this work.
MCC is generally assumed to be a chemotherapy-sensitive tumour, based on its morphological and immunohistochemical similarities with other neuroendocrine malignancies, which is confirmed by an objective response of 63% to first-line chemotherapy in our series. Comparable results were obtained in a retrospective study by Voog et al., who reported responses in MCC in 69% and 57%, respectively [15]. In a review of 204 patients given chemotherapy [16], similar response rates were reported, namely 59% for patients with distant metastasis and 68% for cases with locally advanced disease. Iyer et al. reported response rates of 55% in first line and 23% in second line [17]. Another retrospective study reported response rates of 29% and 20%, respectively [18]. The lower RR in first line in this study is at least in part attributable to the inclusion of non-evaluable patients and high median age (78 years).
In our series, combinations of platinum salts and etoposide were the most commonly used regimen, with responses seen in 69% of the treated patients. This is similar to responses reported by Iyer et al. with this regimen (60.5%). Fenig et al., [3] reported a response rate of 60% to such treatment in 10 patients. According to the literature, combinations of etoposide with platinum are commonly used for MCC, with reasonable efficacy and tolerability [19]. In another retrospective study, a combination of cisplatin and doxorubicin was found to be superior to other regimens in terms of response rate [15]. A higher response rate was also seen with regimens that included 5-fluorouracil as compared to schedules without a fluoropyrimidine [15]. The French Society of Dermatology proposed to use combinations of carboplatin plus etoposide or cyclophosphamide, doxorubicin and vincristine for the treatment of advanced MCC [20], supported by some American and German guidelines [21,22]. In our series, combination chemotherapy was associated with superior response rates compared to single agents with 69% versus 33% respectively. Since MCC occurs mainly in the elderly, not all patients however qualify for combination schedules due to age or co-morbidity. Combination chemotherapy is associated with more severe adverse events and a higher incidence of toxic death compared to single agents. In our series, three treatment-associated deaths were reported, but overall survival still compares well with some published data.
Patient 80374358 was a male with diagnosis of MCC with synchronous liver metastasis at the age of 65 years. The patient had no relevant comorbidity at initial diagnosis. In total he received three lines of systemic treatment: cisplatin/etoposide/ifosfamide, doxorubicin/etoposide and irinotecan. He died during chemotherapy with the topoisomerase inhibitor due to severe diarrhea, vomiting and hypovolemic shock.
Patient 61550430 was a 74 year-old male with the diagnosis of MCC with pelvic lymph node metastasis at first diagnosis. He had a history of arterial hypertension and a history of locally advanced bladder cancer. He had extensive radiotherapy treatment and his systemic therapy was restricted to one line of carboplatin and etoposide. The patient had disease progression during this systemic treatment, complicated by severe diarrhea, acute renal insufficiency and a severe urinary track infection with E. coli. He died two months after terminating his systemic chemotherapy.
Patient 82717703 was a 53 year-old female with diagnosis of MCC. At first diagnosis she had diffuse axillary lymph node and hepatic metastasis. She had a history of kidney transplantation due to Alport syndrome. In addition she had insulin-dependent diabetes mellitus and a secondary Cushing syndrome. For MCC she received two lines of chemotherapy, carboplatin/etoposide complicated by a deterioration of the function of her transplant kidney, and gemcitabine/docetaxel. During second line treatment she developed an E. coli urosepsis and severe thrombocytopenia. She died within 2 weeks of her last contact in the hospital due to an unknown cause.
Especially among older patients and those with poorer performance or organ dysfunction treatment with a single agent can be considered, although possibly less effective. As an example, orally administered etoposide is well-tolerated and can be applied on an outpatient basis. Complete responses were reported in 3 out of 4 patients in a small series [23].
Our data highlight once again the “responding resistance” pattern seen in clinical routine in different neuroendocrine malignancies. While initial response rates are high, the median duration of response in our series was short and the overall survival far from being satisfactory. We observed a median progression-free survival after first-line chemotherapy of 8 months (range 1-146), which again is comparable to published results [15,17]. The median overall survival since the start of chemotherapy was 13 months. The 2- and 5-year overall survival rates were only 59% and 29%, respectively. This matches the experience of Tai et al., [16], who reported survival of 59% at 2 years and 22% at 5 years. Chemotherapy results reported from our other series are similar to what can be achieved in other neuroendocrine carcinomas, such as small cell lung cancer or high grade gastroenteropancreatic neuroendocrine carcinomas. In these tumor types, response rates vary between 33 and 67% [16] and progression-free survival ranges between 1 and 21 months [24]. Our monocentric experience provides supportive evidence for the effectiveness of chemotherapy in the treatment of MCC, but also highlights the short duration of clinical benefit in this rare disease.
In contrast with low to intermediate grade neuroendocrine tumors, the use of targeted agents such as tyrosine kinase inhibitors has not become part of the routine treatment of patients with MCC. Only a limited number of studies have assessed the potential role of these molecules in MCC. Most of the available data are based on case reports. MCC is associated with an overexpression of vascular endothelial growth factor receptor, platelet derived growth factor receptor and KIT, which all belong to the same family of tyrosine kinase receptors [8,25]. In a clinical trial with pazopanib, a multi-targeted tyrosine kinase inhibitor, a patient with metastatic MCC showed an impressive tumor regression, but progression-free survival was short [18]. A trial with imatinib in MCC had to be discontinued due to the lack of activity [26-28], even though some case reports suggested therapeutic effects [29-31]. While the use of mammalian target of rapamycin inhibitors has become common practice in other neuroendocrine malignancies [32], they are not used on a routine basis in MCC. Only individual patients in our series were treated with everolimus, without relevant clinical benefit.
Not all patients with MCC qualify for modern treatment with immune checkpoint inhibitors, for example individuals with a history of autoimmune diseases or organ transplant recipients or other patients who require therapy with immunosuppressive agents. For this subset of patients the results achieved with chemotherapy in the pre-immunotherapy era as summarized here may be very important, as chemotherapy maybe a reasonable alternative to immune checkpoint inhibition.
An International Workshop on Merkel Cell Carcinoma Research was held at the National Cancer Institute in 2018, resulting in an expert consensus statement on the biological features, clinical presentation, prognosis and management of this uncommon cancer [33].
Conclusion and Outlook
MCC is an aggressive neuroendocrine tumor of the skin with a high risk of local relapse and development of metastatic spread. Patients with disseminated disease have a reasonable chance to respond to traditional cytotoxic chemotherapy, but the quality and duration of response is poor even if potentially toxic platinum-based schedules are used. The orphan character of this malignancy made it difficult in the past to perform prospective, ideally comparative clinical trials, resulting in a lack of standardization of the treatment for patients with advanced disease. While chemotherapy remains an important component, there is a high unmet medical need to develop safer and more efficient treatment alternatives that can be used either in combination with chemotherapy or in a sequential manner. Ideally such agents should affect tumor cells through different mechanisms than conventional cytotoxic agents, as we are failing to gain durable disease control with chemotherapy alone [18,22].
Like other virus-associated cancers, MCC has a high expression of PD-L1 in tumor cells and in tumor infiltrating lymphocytes. Moreover MCV-positive tumors express high levels of viral oncoproteins and MCV-negative tumors bear a high mutational burden. These are two biological features commonly known to serve as immunotherapeutic targets [11]. This rationale and the recent exploration of immune checkpoint modulators in multiple common and rare solid tumors led to new avenues for treatment of patients with advanced MCC. Avelumab, a PD-L1 antibody, has recently been approved for the treatment of metastatic MCC, based on an uncontrolled phase II trial. This study reported a response rate of 33% in a pretreated population, with some of these responses being durable. The documented median overall survival was 12.9 months. This is similar to what we observed in our series with first line chemotherapy. This suggests that avelumab may be at least as effective, and should be tested in a controlled trial in first line, particularly because as overall survival curve suggests that avelumab may induce long term survival in approximately one third of treated patients [35]. Remarkable responses have been observed with PD-1 antibodies as well [36]. A phase II trial with pembrolizumab as first line treatment showed a response rate of 56%, with a PFS beyond 6 months [37]. A number of other trials are currently exploring these and other immune checkpoint inhibitors or alternative immunotherapeutic concepts in this disease. Current guidelines recommend immune checkpoint inhibitors based on the available clinical data and the good biological rationale for this treatment strategy. Immune therapy is a reasonable alternative for cytotoxic chemotherapy in advanced MCC. Future clinical research will explore the potential of combining immune checkpoint inhibitor therapy with other treatment modalities. These are likely combinations of different immune checkpoint inhibitors but one could also envision combinations with cytotoxic chemotherapy as currently explored in lung cancer.
Data from the metastatic setting should also be used to develop more efficient treatment strategies for locally advanced MCC. Given the aggressive pattern of dissemination of this disease, MCC has to be regarded as a systemic malignancy and patients could potentially benefit from multimodal treatment approaches in early stage disease. To improve cure rates and overall survival we could envision perioperative treatment approaches incorporating (neo) adjuvant chemotherapy +/- immunotherapy in the local treatment of MCC in clinical trials.
Due to the rarity of MCC trials will likely follow the path of studies conducted in more prevalent diseases. Due to some unique immunological features of MCC immune checkpoint inhibitors are likely to play a central role in future systemic therapy. MCC can function as a model disease for novel immunotherapy concepts, and this rare entity should no longer be neglected when exploring novel immune therapies in multi-tumor basket trials.
Declaration of Interest
Patrick Schöffski: none; Gitte Moors: none; Paul Clement: NIH (patents/royalties/other intellectual property (institutional)); Abbvie, Vifor, Leo Pharma, BMS (Consulting or advisory role (institutional)); speakers’ bureau by Astra Zeneca, BMS, Merck (institutional); Herlinde Dumez: Astellas, Novartis, Bayer, Sanofi, Roche, Astra-Zeneca, Ipsen; Janssen-Cilag: Pfizer and MSD – travel grant; Oliver Bechter: none.
REFERENCES
- Bichakjian CK, Lowe L, Lao CD, Sandler HM, Bradford CR, Johnson TM, et al. (2007) Merkel cell carcinoma: Critical review with guidelines for multidisciplinary management. Cancer 110(1): 1-12.
- Hodgson HC (2005) Merkel cell carcinoma: Changing incidence trends. J Surg Oncol 89(1): 1-4.
- Fenig E, Brenner B, Katz A, Rakovsky E, Hana MB, Sulkes A (1997). The role of radiation therapy and chemotherapy in the treatment of Merkel cell carcinoma. Cancer 80(5): 881-885.
- Gessner K, Wichmann G, Boehm A, Reiche A, Bertolini J, Brus J, et al. (2011) Therapeutic options for treatment of Merkel cell carcinoma. Eur Arch Otorhinolaryngol 268(3): 443-448.
- Agelli M, Clegg LX, Becker JC, Rollison DE (2010) The etiology and epidemiology of Merkel cell carcinoma. Curr Probl Cancer 34(1): 14-37.
- Poulsen M (2004) Merkel-cell carcinoma of the skin. Lancet Oncol 5(10): 593-599.
- Pectasides D, Pectasides M, Economopoulos T (2006) Merkel cell cancer of the skin. Ann Oncol 17(10): 1489-1495.
- Desch L, Kunstfeld R (2013) Merkel cell carcinoma: Chemotherapy and emerging new therapeutic options. J Skin Cancer 2013: 327150.
- Engels EA, Frisch M, Goedert JJ, Biggar RJ, Miller RW (2002) Merkel cell carcinoma and HIV infection. Lancet 359(9305): 497-498.
- Penn I, First MR (1999) Merkel's cell carcinoma in organ recipients: report of 41 cases. Transplantation 68(11): 1717-1721.
- Tello TL, Coggshall K, Yom SS, Yu SS (2018) Merkel cell carcinoma: An update and review: Current and future therapy. J Am Acad Dermatol 78(3): 445-454.
- Kline L, Coldiron B (2016) Mohs micrographic surgery for the treatment of Merkel cell carcinoma. Dermatol Surg 42(8): 945-951.
- Mojica P, Smith D, Ellenhorn JD (2007) Adjuvant radiation therapy is associated with improved survival in Merkel cell carcinoma of the skin. J Clin Oncol 25(9): 1043-1047.
- Chen MM, Roman SA, Sosa JA, Judson BL (2015) The role of adjuvant therapy in the management of head and neck Merkel cell carcinoma: An analysis of 4815 patients. JAMA Otolaryngol Head Neck Surg 141(2): 137-141.
- Voog E, Biron P, Martin JP, Blay JY (1999) Chemotherapy for patients with locally advanced or metastatic Merkel cell carcinoma. Cancer 85(12): 2589-2595.
- Tai P, Yu E, Winquist E, Hammond A, Stitt L, Tonita J, et al. (2000) Chemotherapy in neuroendocrine/Merkel cell carcinoma of the skin: Case series and review of 204 cases. J Clin Oncol 18(12): 2493-2499.
- Iyer JG, Blom A, Doumani R, Lewis C, Tarabadkar ES, Anderson A, et al. (2016) Response rates and durability of chemotherapy among 62 patients with metastatic Merkel cell carcinoma. Cancer Med 5(9): 2294-2301.
- Cowey CL, Mahnke L, Espirito J, Helwig C, Oksen D, Bharmal M (2017) Real-world treatment outcomes in patients with metastatic Merkel cell carcinoma treated with chemotherapy in the USA. Future Oncol 13(19): 1699-1710.
- Davis MP, Miller EM, Rau RC, Johnson OE, Naille RA, Crnkovich MJ (1990) The use of VP16 and cisplatin in the treatment of Merkel cell carcinoma. J Dermatol Surg Oncol 16(3): 276-278.
- Boccara O, Girard C, Mortier L, Bens G, Saiag P, Guillot B, et al. (2012) Guidelines for the diagnosis and treatment of Merkel cell carcinoma-Cutaneous Oncology Group of the French Society of Dermatology. Eur J Dermatol 22(3): 375-379.
- Miller SJ, Alam M, Andersen J, Berg D, Bichakjian CK, Bowen G, et al. (2009) NCCN clinical practice guidelines in oncology: Merkel cell carcinoma. J Natl Compr Canc Netw 7(3): 322-332.
- Becker J, Mauch C, Kortmann RD, Keilholz U, Bootz F, Garbe C, et al. (2008) Onkologische Leitlinie kutanes neuroendokrines Karzinom (Merkelkarzinom). J Dtsch Dermatol Ges 6: S15-S16.
- Schlaak M, Podewski T, Von Bartenwerffer W, Kreuzberg N, Bangard C, Mausch C et al. (2012) Induction of durable responses by oral etoposide monochemotherapy in patients with metastatic Merkel cell carcinoma. Eur J Dermatol 22(2): 187-191.
- Weber CH (2013) Medical treatment of neuroendocrine tumours. Curr Opin Endocrinol Diabetes Obes 20(1): 27-31.
- Fernández-Figueras MT, Puig L, Musulén E, Gilaberte M, Lerma E, Serrano S, et al. (2007) Expression profiles associated with aggressive behavior in Merkel cell carcinoma. Mod Pathol 20(1): 90-101.
- Davids MS, Charlton A, Ng SS, Chong ML, Laubscher K, Dar M, et al. (2009) Response to a novel multitargeted tyrosine kinase inhibitor pazopanib in metastatic Merkel cell carcinoma. J Clin Oncol 27(26): e97-100.
- Peuvrel L, Quereux G, Brocard A, Renaut JJ, Dreno B (2011) Treatment of a multicentric Merkel cell carcinoma using imatinib. Eur J Dermatol 26(6): 1009-1010.
- Samlowski WE, Moon J, Tuthill RJ, Heinrich MC, Balzer-Haas NS, Merl SA, et al. (2010) A phase II trial of imatinib mesylate in merkel cell carcinoma (neuroendocrine carcinoma of the skin): A Southwest Oncology Group study (S0331). Am J Clin Oncol, 33(5): 495-499.
- Loader DE, Feldmann R, Baumgartner M, Breier F, Schrama D, Becker JC, et al. (2013) Clinical remission of Merkel cell carcinoma after treatment with imatinib. J Am Acad Dermatol 69(4): e181-183.
- Schrama D, Ugurel S, Becker JC (2012) Merkel cell carcinoma: Recent insights and new treatment options. Curr Opin Oncol 24(2): 141-149.
- Weber HC (2013) Medical treatment of neuroendocrine tumours. Curr Opin Endocrinol Diabetes Obes, 20(1): 27-31.
- Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. (2011) Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 364(6): 501-513.
- Harms PW, Harms KL, Moore PS, DeCaprio JA, Nghiem P, Wong MK, et al. (2018) The biology and treatment of Merkel cell carcinoma: Current understanding and research priorities. Nat Rev Clin Oncol 15: 763-776.
- Becker JC, Lorenz E, Ugurel S, Eigentler TK, Kiecker F, Pfohler C, et al. (2017) Evaluation of real-world treatment outcomes in patients with distant metastatic Merkel cell carcinoma following second-line chemotherapy in Europe. Oncotarget 8(45): 79731-79741.
- Kaufman HL, Russell JS, Hamid O, Bhatia S, Terheyden P, D’Angelo SP, et al. (2018) Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer 6(1): 7.
- Winkler JK, Bender C, Kratochwil C, Enk A, Hassel JC (2017) PD-1 blockade: A therapeutic option for treatment of metastatic Merkel cell carcinoma. Br J Dermatol 176(1): 216-219.
- Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, et al. (2016) PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med 374(26): 2542-2552.
Copyright: Schöffski P, et al. ©2019. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Schöffski P (2019). Palliative Systemic Treatment of Advanced Merkel Cell Carcinoma in the Pre-Immunotherapy Era: A Retrospective, Single-Center Analysis of Patients with an Orphan Neuroendocrine Malignancy. Oncogen 2(3): 14.
 Abstract
Abstract  PDF
PDF